# Day -4 Glimpses, 5th March 2022
# Session 1 on Australia Regulatory guideline for filing: General Overview by Mr. Pranav Pandya, Deputy General Manager, Regulatory Affairs, Sun Pharmaceutical Industries Ltd., Vadodara
# Session 2 on eCTD: Module 5 – Clinical study report and Module 2 related to Clinical study report summary by Dr. Haresh Patel, Senior Manager, Sun Pharmaceutical Industries, Vadodara, Gujarat, India.
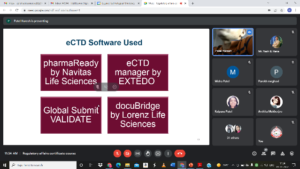
# Session 3 on eCTD software training – EU publishing by Ms. Sheetal Kadam Subject Matter Expert- Regulatory Affairs Solution, Sarjen systems Pvt. Ltd. Ahmedabad, India.