# Day -2 Glimpses, 19th February 2022
# Session 1 on Drug Development Process to Post-marketing surveillance: Regulatory perspective by Dr. Ashutosh Jani Head- Clinical Operations Department Veeda Clinical Research, Ahmedabad
# Session 2 on Drug Master File and CEP: Certificate of Suitability by Ms. Dipa Mehta
Manager of Pharmazone, Ahmedabad
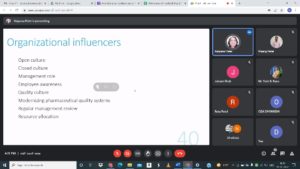
# Session 3 on Key aspects of Data Integrity and its management by Dr. Kalpana Patel
Professor, Anand Pharmacy College, Anand.